You are seeing a free-to-access but limited selection of the activity Altmetric has collected about this research output.
Click here to find out more.
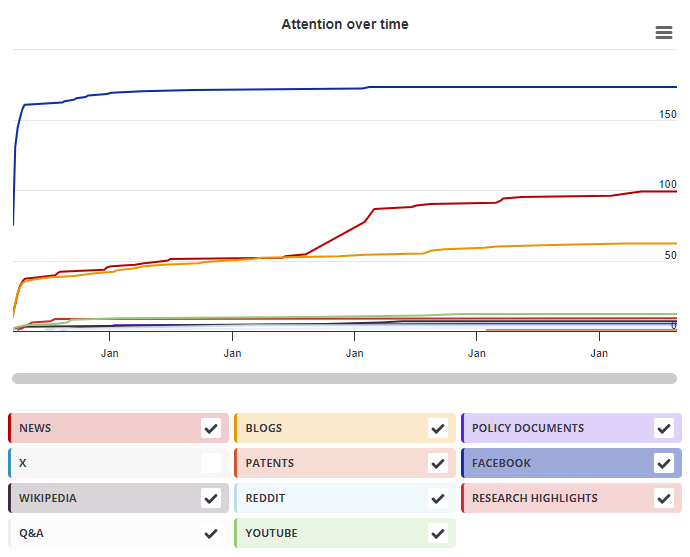
Timeline
Mendeley readers
| Chapter title |
Ethics: Institutional Review Board/Independent Ethics Committee (IRB/IEC)
|
|---|---|
| Chapter number | 21 |
| Book title |
Quick Guide to Good Clinical Practice
|
| Published by |
Springer, Cham, January 2017
|
| DOI | 10.1007/978-3-319-44344-7_21 |
| Book ISBNs |
978-3-31-944343-0, 978-3-31-944344-7
|
| Authors |
Cemal Cingi, Nuray Bayar Muluk, Cingi, Cemal, Muluk, Nuray Bayar |

Mendeley readers
The data shown below were compiled from readership statistics for 14 Mendeley readers of this research output. Click here to see the associated Mendeley record.
Geographical breakdown
| Country | Count | As % |
|---|---|---|
| Unknown | 14 | 100% |
Demographic breakdown
| Readers by professional status | Count | As % |
|---|---|---|
| Student > Bachelor | 3 | 21% |
| Student > Ph. D. Student | 3 | 21% |
| Student > Master | 2 | 14% |
| Professor > Associate Professor | 1 | 7% |
| Student > Postgraduate | 1 | 7% |
| Other | 0 | 0% |
| Unknown | 4 | 29% |
| Readers by discipline | Count | As % |
|---|---|---|
| Nursing and Health Professions | 2 | 14% |
| Pharmacology, Toxicology and Pharmaceutical Science | 1 | 7% |
| Biochemistry, Genetics and Molecular Biology | 1 | 7% |
| Linguistics | 1 | 7% |
| Arts and Humanities | 1 | 7% |
| Other | 4 | 29% |
| Unknown | 4 | 29% |
